27 Internal Loops
27.1 1×1, 1×2, 2×2 Internal Loops
Small symmetric internal loops have tabulated free energy and enthalpy changes, where experimentally determined values are used if available.
27.2 Other Internal Loops
The stabilities of other internal loops are predicted using the equation:
ΔG°37 internal = ΔG°37 initiation(n) + ΔG°37 asymmetry × |n1 – n2| + ΔG°37 mismatch(mismatch 1) + ΔG°37 mismatch(mismatch 2)
where the initiation is a length dependent term for n unpaired nucleotides, an asymmetry term is multiplied by the absolute value of the difference in the number of unpaired nucleotides on each side of the loop, and sequence-dependent mismatch terms are applied for first mismatches of specific sequences.
Similarly, the enthalpy change is predicted with the equation:
ΔH°internal = ΔH°initiation(n) + ΔH°asymmetry × |n1 – n2| + ΔH°mismatch(mismatch 1) + ΔH°mismatch(mismatch 2) + ΔH°AT closure(per AT)
where terms are analagous to those for predicting folding free energy changes.
The mismatch parameters are sequence-dependent and are different for 1×(n-1) loop, 2×3 loop, and other internal loops. In the case of 1×(n–1) loops, the mismatches are set to 0 kcal/mol for free energy and enthalpy changes.
27.3 Examples
2×2 internal loop

ΔG°37 = ΔG°37(Watson-Crick-Franklin Helix) + ΔG°37 intermolecular initiation + ΔG°37(Internal Loop)
ΔG°37 = ΔG°37(CG followed by AT) + ΔG°37(CG followed by GC) + ΔG°37 intermolecular initiation + ΔG°37(2×2 Internal Loop)
ΔG°37 = -1.5 kcal/mol - 2.2 kcal/mol + 1.0 kcal/mol + 1.6 kcal/mol
ΔG°37 = -1.1 kcal/mol
ΔH° = ΔH°(Watson-Crick-Franklin Helix) + ΔH°intermolecular initiation + ΔH°(Internal Loop)
ΔH °= ΔH°(CG followed by AT) + ΔH°(CG followed by GC) + ΔH°intermolecular initiation + ΔH°(2×2 Internal Loop)
ΔH° = -9.9 kcal/mol - 9.8 kcal/mol - 7.2 kcal/mol - 2.2 kcal/mol
ΔH° = -29.1 kcal/mol
Note that the internal loop lookup tables account for terminal AT pairs that are adjacent to internal loops.
1×5 internal loop
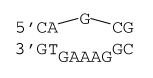
ΔG°37 = ΔG°37(Watson-Crick-Franklin Helix) + ΔG°37 intermolecular initiation + ΔG°37(Internal Loop)
ΔG°37 = ΔG°37(CG followed by AT) + ΔG°37(CG followed by GC) + ΔG°37 intermolecular initiation + ΔG°37 initiation(6) + ΔG°37 asymmetry×|n1 - n2| + ΔG°37 mismatch(mismatch 1) + ΔG°37 mismatch(mismatch 2)
ΔG°37 = -1.5 kcal/mol - 2.2 kcal/mol + 1.0 kcal/mol + 3.9 kcal/mol + .4×|1-5| kcal/mol + 0 kcal/mol + 0 kcal/mol
ΔG°37 = 2.8 kcal/mol
ΔH° = ΔH°(Watson-Crick-Franklin Helix) + ΔH°intermolecular initiation + ΔH°(Internal Loop)
ΔH°= ΔH°(CG followed by AT) + ΔH°(CG followed by GC) + ΔH°intermolecular initiation + ΔH°initiation(6) + ΔH°asymmetry × |n1 - n2| + ΔH°mismatch(mismatch 1) + ΔH°mismatch(mismatch 2) + ΔH° AT closure
ΔH° = -9.9 kcal/mol - 9.8 kcal/mol - 7.2 kcal/mol + 0.0 kcal/mol + 0.0 × |1-5| kcal/mol + 0.0 kcal/mol + 0.0 kcal/mol + 3.2 kcal/mol
ΔH° = -23.7 kcal/mol
Note that the free energy and enthalpy changes for first mismatches in 1×(n-1) internal loops are 0 kcal/mol.
2×3 internal loop with stabilizing mismatches
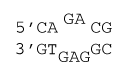
ΔG°37 = ΔG°37(Watson-Crick-Franklin Helix) + ΔG°37 intermolecular initiation + ΔG°37(Internal Loop)
ΔG°37 = ΔG°37(CG followed by AT) + ΔG°37(CG followed by GC) + ΔG°37 intermolecular initiation + ΔG°37 initiation(5) + ΔG°37 asymmetry×|n1 - n2| + ΔG°37 mismatch(mismatch 1) + ΔG°37 mismatch(mismatch 2)
ΔG°37 = -1.5 kcal/mol - 2.2 kcal/mol + 1.0 kcal/mol + 2.0 kcal/mol + .4×|3 - 2| kcal/mol - 0.4 kcal/mol - 1.0 kcal/mol
ΔG°37 = -1.7 kcal/mol
ΔH° = ΔH°(Watson-Crick-Franklin Helix) + ΔH°intermolecular initiation + ΔH°(Internal Loop)
ΔH°= ΔH°(CG followed by AT) + ΔH°(CG followed by GC) + ΔH°intermolecular initiation + ΔH°initiation(5) + ΔH°asymmetry × |n1 - n2| + ΔH°mismatch(mismatch 1) + ΔH°mismatch(mismatch 2) + ΔH° AT closure
ΔH° = -9.9 kcal/mol - 9.8 kcal/mol - 7.2 kcal/mol + 0.0 kcal/mol + 0.0×|3 - 2| kcal/mol + 1.5 kcal/mol - 6.2 kcal/mol + 3.2 kcal/mol
ΔH° = -28.4 kcal/mol
27.4 Parameter Tables
1×1 internal loop free energy change tables are available in text and html format. Enthalpy change tables are available in text and html format. Note that these tables incorporate the AT closure penalties and therefore no AT helix end penalty should be applied for internal loop closure.
1×2 internal loop free energy change tables are available in text and html format. Enthalpy change tables are available in text and html format. Note that these tables incorporate the AT closure penalties and therefore no AT helix end penalty should be applied for internal loop closure.
2×2 internal loop free energy change tables are available in text and html format. Enthalpy change tables are available in text and html format. Note that these tables incorporate the AT closure penalties and therefore no AT helix end penalty should be applied for internal loop closure.
