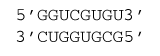
∆G°37 = ∆G°37
intermolecular initiation + ∆G°37 GU end penalty + ∆G°37(GC
followed by GU, followed by UG, followed by GC) + ∆G°37(CG
followed by GU) + ∆G°37(GU
followed by UG) + ∆G°37(UG
followed by GC) + ∆G°37(GC
followed by UG)
∆G°37 = 4.09
kcal/mol + 0.45
kcal/mol – 4.12
kcal/mol –1.41 kcal/mol + 1.29 kcal/mol – 1.41 kcal/mol – 2.51 kcal/mol
∆G°37 = –3.62 kcal/mol
∆H° = ∆H°intermolecular initiation + ∆H°GU end penalty+ ∆H°(GC
followed by GU, followed by UG, followed by GC) + ∆H°(CG
followed by GU) + ∆H°(GU
followed by UG) + ∆H°(UG
followed by GC) + ∆H°(GC
followed by UG)
∆H° = 3.61
kcal/mol + 3.72
kcal/mol – 30.80
kcal/mol – 5.61 kcal/mol – 14.59 kcal/mol – 5.61 kcal/mol – 12.59 kcal/mol
∆H° = –61.87 kcal/mol
Note that
this example shows the stack of GU followed by UG in two different contexts, including the stabilizing context and the destabilizing context.
 Turner 2004
Turner 2004